Disease Creep: How we're fooled into using more medicine than we need.
Published: December 22, 2011 updated 12/24/2017
When doctors recommend tests, drugs or surgeries to prevent bad outcomes (think cholesterol-lowering agents to prevent strokes or cardiac stents to prevent heart attacks) they tap into our deepest sense of what constitutes common sense: An ounce of prevention. Catch it early. A stitch in time.
It can’t be a bad thing to catch problems early, can it?
Unfortunately, one of the toughest things to explain is why detecting some illnesses at their earliest stages can cause more harm than good. Take this example: Since elevated cholesterol is associated with a higher risk of cardiovascular disease, doctors often prescribe drugs known as statins to people with elevated cholesterol levels in the hopes of reducing their risk of a heart attack or stroke.
Here comes the part that’s tough to explain – because it is so counterintuitive: Statins only help individuals who already have had a heart attack or stroke (with a few exceptions, and more on that later).
Of course, this makes no sense to most people. Isn’t the whole point of taking cholesterol-lowering agents to prevent a heart attack? Why should anyone wait until after a heart attack or stroke to begin taking a drug designed to prevent a heart attack or stroke?
The answer rests with disease creep and the simple statistical quirks that come with it. In the past, doctors treated diseases that caused symptoms. But now we have tests and imaging machines that can detect risk factors and illnesses in their earliest stages. Like cholesterol. Elevated cholesterol is not a disease. It doesn’t cause symptoms. It is a risk factor. People with high cholesterol levels are somewhat more likely to develop a heart attack or stroke, but they are at far less risk than individuals who already have cardiovascular disease. This is the definition of disease creep: when pre-conditions or risk factors are treated as if they are the same as the actual disease state.
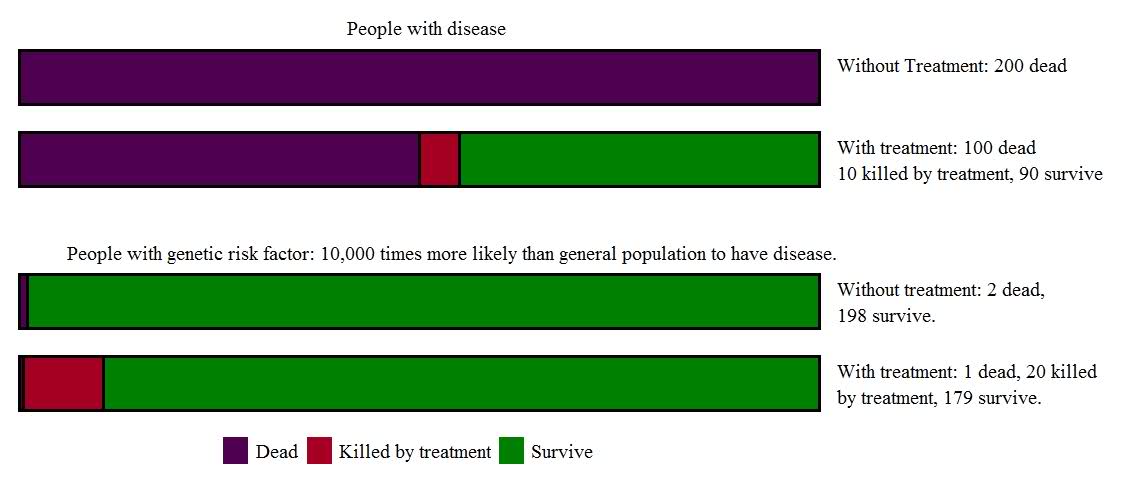
Here’s a thought experiment (with purposefully exaggerated numbers) to help understand this puzzle: Imagine a group of people who have the rare but awful Disease A, which is so terrible that all of its victims will die. Now imagine the discovery of Wonder Drug X, which cures half of the patients with Disease A. Unfortunately, Wonder Drug X does have a pretty bad side effect profile – it’s a very powerful drug, after all – and 10 percent of people who take it will die from liver failure. Despite this worrisome side effect, Wonder Drug X is truly an advance for patients with Disease A: For every 200 patients with the disease who are treated, 100 will now survive and only 10 of the 100 survivors will die of the drug’s side effects. That means 90 more people out of 200 will survive thanks to Wonder Drug X.
But now imagine a different group of 200 people, who don’t actually have disease A, but instead have a genetic marker which “is associated with” Disease A. In this scenario only 1 in a million people in the general population will get disease A. If you have the genetic marker, the risk is much higher, such that 2 of these 200 people will develop the disease at some time in the future. The genetic test gets highly promoted – “find out your risk early, because we now have a treatment that works, and the sooner you’re treated, the better!” There is a tiny grain of truth to this – of the 2 people identified by the genetic test, 1 (50%) will now be saved by Wonder Drug A. This might sound just as good as before; here’s a group of people with 10,000 times (!) the risk of the general population to develop a uniformly fatal disease. Surely it’s worth taking a drug that can cure that disease in half the cases, isn’t it?
But it really isn’t the same. Because saving that one life isn’t remotely worth the harm caused by Wonder Drug X in these 200 people. For while each individual is at a 10,000 fold risk of the disease, only 2 are destined to die from it. On the other hand, if they take Wonder Drug X, roughly 20 (10 percent of the 199 survivors) will die unnecessarily of liver failure.
In the group who already have Disease A, Wonder Drug X is great, saving 100 lives and killing 10, for a net benefit of 90 lives saved. But this contrasts with those who are “caught early” and treated because of a risk factor (before they actually have the disease); in this instance, Wonder Drug X is a disaster, saving 1 life and killing 20, for a net harm of 19 extra deaths.
Most people with high cholesterol are like our fictional group of people with the genetic marker for Disease A; they have a risk factor for a disease, but not the disease itself. Most individuals with high cholesterol but without known heart disease will not die of an early heart attack or stroke. As with Disease A and Wonder Drug X, the ratio of benefit to harm that will result from treatment, is (ironically) much lessfavorable in these people (not yet “patients”) who are simply “at risk of getting the disease” than it is among people who actually already have it.
The big difference between Wonder Drug X and statins is that cholesterol-lowering drugs are neither as beneficial, nor as harmful as Wonder Drug X; for low-risk individuals, the harms of statins appear simply to balance their benefits. The primary downside is the billions of dollars, lost time and inconvenience caused by the attempt to treat disease before it happens. Of course there’s one other harm: our misdirected attention. It turns out that compared to taking a statin, exercising is as effective as taking a statin for preventing heart disease and death, and it comes without nearly the same cost, or the same adverse side effects.
In 2003 and 2010 the widely respected and independent group Therapeutics Initiative, published in-depth analyses of randomized controlled trials of statins for low-risk individuals (called primary prevention) and concluded that for this population, “Statins do not have a proven net health benefit.” Yet despite the lack of proven benefit, the overwhelming majority (up to three quarters ) of people taking the drugs are low risk. In other words, “statins do not have a proven net health benefit” for most of the people who are taking them.
Using the National Cholesterol Education Project guidelines, researchers determined that vast numbers of people on statins are overtreated. (What’s really crazy is that among the far smaller group of high-risk patients, most were undertreated.) For an incisive analysis of the studies and how the majority of patients on statins are overtreated, a review in the Lancet is illuminating. (Even worse than our overtreatment of adults with high cholesterol is the new evidence-free movement to start trying to lower cholesterol levels in kids. Christie Aschwanden deconstructs that idea and the conflicts of interest behind it in her recent piece at Slate.)
Disease creep, or our drive to catch disease early, and the counterintuitive nature of waiting for symptoms, are pushing more and more of us onto medicines we may not need. In addition to “pre-heart disease,” which is in effect what having elevated cholesterol amounts to, we now have pre-hypertension and pre-diabetes. We catch cancers too early to know if they are ever going to cause a problem. And we put a million stents a year in people’s hearts – yet elective stent placement offers no benefitat all in terms of reduced heart attacks or deaths. Nearly half the population in the U.S. is taking at least one prescribed drug, according to the Centers for Disease Control and Prevention. More than one in ten is taking five or more medicines. Yet we are sicker and don’t live as long as people in Canada or England – or 35 other nations where they generally take fewer medicines and undergo fewer tests.
Now that the top-selling drug in the world, Lipitor, is going off patent after 17 years on the market, its manufacturer is already poised to keeps its sales high by promoting its drug directly to you, the consumer.
Don’t be distracted by all the drug ads and medical news: by far the most effective way to prevent heart disease (as well as diabetes, stroke, hypertension, etc.) is to resist being overweight, exercise routinely, and don’t smoke. That’s a prescription for health that won’t cause fatigue, nausea or death from liver failure.
See also:
1. Redberg RF, Katz MH. Statins for primary prevention: The debate is intense, but the data are weak. JAMA Internal Medicine. 2016. http://dx.doi.org/10.1001/jamainternmed.2016.7585.
2. Abramson J, Redberg RF. Don't Give More Patients Statins. New York Times2013. http://www.nytimes.com/2013/11/14/opinion/dont-give-more-patients-statins.html?_r=0.
3. Ioannidis JP. More Than a Billion People Taking Statins?: Potential Implications of the New Cardiovascular Guidelines. JAMA. Dec 2, 2013. http://www.ncbi.nlm.nih.gov/pubmed/24296612.
4. Abramson JD, Rosenberg HG, Jewell N, Wright JM. Should people at low risk of cardiovascular disease take a statin? BMJ. 2013;347:f6123. http://www.ncbi.nlm.nih.gov/pubmed/24149819.
5. Newman DH, Saini V, Brody H, et al. Statins for people at low risk of cardiovascular disease. Lancet. Nov 24, 2012;380(9856):1814; author reply 1817-1818. http://www.ncbi.nlm.nih.gov/pubmed/23177692.
6. Redberg RF. Reassessing Benefits and Risks of Statins. New England Journal of Medicine. 2012;367(8):776-776. http://www.nejm.org/doi/full/10.1056/NEJMc1207079.
7. Wright JM. Do statins have a role in primary prevention? An update.2010. http://www.ti.ubc.ca/sites/ti.ubc.ca/files/77.pdf.
8. Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: The Allhat-LLT randomized clinical trial. JAMA Internal Medicine. 2017;177(7):955-965. http://dx.doi.org/10.1001/jamainternmed.2017.1442